Бактерии 1-2. Триптофан стимулирует синтез иук до количеств 40 мкгмл культуральной жидкости, однако максимальное количество зависит от особенностей штамма
 Скачать 105.45 Kb. Скачать 105.45 Kb.
|
1 2 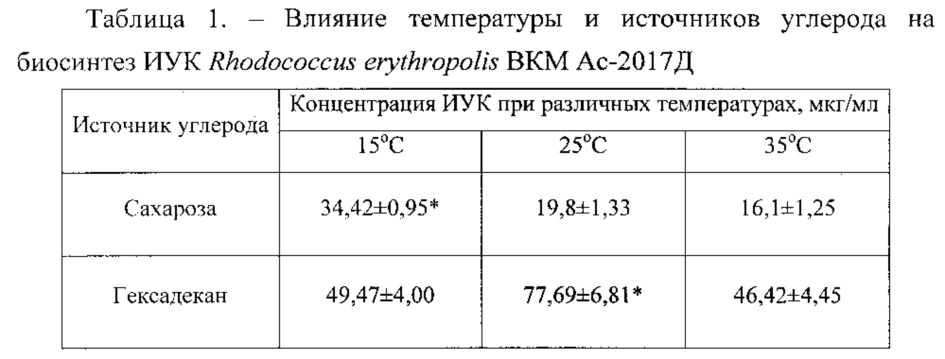 Если источником углерода выступала сахароза, то максимальная концентрация фитогормона в 34,42 мкг/мл наблюдалась при температуре 15°С; на гексадекане- отмечена при температуре 25°С и составила 77,69 мкг/мл. Литература 1. Phillips Theresa M., Seech Alan G., Hung Lee, Trevors Jack T. Biodegradation of hexachlorocyclohexane (HCH) by microorganisms // Biodegradation. – 2005, V. 16, Issue 4, – P. 363-392. 2. Кулаева О.Н., Кузнецов В.В. Новейшие достижения и перспективы в области изучения цитокининов// Физиология растений. –2002. – Т. 49, № 4. – С. 626-640. 3. Lîpez-Bucio J., Campos-Cuevas J.C., Hernàndez-Calderon E., VelàsquezBecerra C., Farias-Rodriguez R., Macías-Rodriguez L.I., Valencia-Cantero E. Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana // Molecular Plant-Microbe Interactions. – 2007. – V. 20, № 2. – P. 207-217. 4. Shishido M.·Chanway C.P. Forest soil community responses to plant growth 4. Смирнов В.В., Киприянова Е.А. Бактерии рода Pseudomonas. – Киев: Наук. думка, 1990. – 264 с. 5. Whipps J.M. Microbial interactions and biocontrol in the rhizosphere // J. Experiment. Botany. 2001. Vol. 52, № 1. P. 487-511. 6. Feklistova I. N., Maksimova N. P. Obtaining Pseudomonas aurantiaca strains capable of overproduction of phenazine antibiotics // Microbiology.2008. Vol. 77., №2. P. 176-180. 7. Феклистова И.Н., Максимова Н.П. Синтез пирролнитрина бактериями Pseudomonas aurantiaca B-162 // Физиологические, биохимические и молекулярные основы функционирования биосистем: Труды Белорусского государственного университета. Т. 3., ч. 1 / под ред. В.М. Юрина. – Минск: 2008. – С. 148–155. 8. Феклистова И.Н., Веремеенко Е.Г. Стимуляция роста растений бактериями Pseudomonas aurantiaca B-162 // Актуальные проблемы изучения фито- и микобиоты: Сб. статей / Под ред. В.Д. Поликсеновой. – Минск: 2004. – С. 203–205. 9. Burr T.J., Schoth M.N., Suslow T. Increased potato yields by treatment of seed pieces with specific strains of Pseudomonas fluorescens and P. putida // Phytopatology. 1978. Vol. 68. № 9. P. 1377-1383. 10. Suslow T.W., Schroth M.N. Rhizobacteria of sugar beet: effects of seed application and root colonization on yield // Phytopatology. 1982. Vol. 72. № 2. P. 199-206. 11. Inoculation of plant growth, yields and physiological properties of tubers in potato and sugar-beet regions J. Vrany, M. Rasochova, A. Fiker e.a. // Folia Microbiol. 1990. Vol. 35. № 2. P. 326-335. 12. Pattern C.L., Glick B.R. Role of Pseudomonas putida idnoleacetic acid in development of the host plant root system // Appl. Environ. Microbiol. 2002. Vol. 68. №. 8. P. 3795-3801. 13. de Bellis P., Ercolani G.L. Growth interactions during bacterial colonization of seedling rootless // Appl. Environ. Microbiol. 2001. Vol. 67. № 4. P. 1945-1948. 14. Хомяк А. И., Асатурова А. М., Сидорова Т. М. Технология получения биофунгицидов на основе новых бактерий рода Bacillus для защиты озимой пшеницы и других сельскохозяйственных культур от экономически значимых болезней. Мат. II науч.-практ. конф. студентов, аспирантов и молодых ученых «Современные аспекты производства и переработки сельскохозяйственной продукции». Краснодар, 2016: 216-224. 15. Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Micro Review. Mol. Microbiol., 2005, 56(4): 845-857. 16. Wang T., Liang Y., Wu M., Chen Z., Lin J., Yang L. Natural products from Bacillus subtilis with antimicrobial properties. Chinese J. Chem. Eng., 2015, 23(4): 744-754. 17. Jacques P. Surfactin and other lipopeptides from Bacillus spp. In: Biosurfactants. Microbiology monographs /G. Soberón-Chávez (ed.). Springer, Berlin, Heidelberg, 2011, V. 20. 18. Nagorska K., Bikowski M., Obuchowski M. Multicellular behavior and production of a wide variety of toxic substances support usage of Bacillus subtilis as a powerful biocontrol agent. Acta Biochim. Pol., 2007, 54(3): 495-508. 19. Lopez D., Fischbach M.A., Chu F., Losick R., Kolter R. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. PNAS USA, 2009, 106: 280-285 (doi: 10.1073/pnas.0810940106). 20. Fickers P., Guez L.-S., Damblon C., Leclérel V., Béchet M., Jacques P., Joris B. High-level biosynthesis of the anteiso-C17 isoform of the antibiotic mycosubtilin in Bacillus subtilis and characterization of its candidacidal activity. Appl. Environ. Microb., 2009, 12: 4636-4640 (doi: 10.1128/AEM.00548-09). 21. Farace G., Fernandez O., Jacquens L., Coutte F., Krier F., Jacques Ph., Clément Ch., Barka E.A., Jacquard C., Dorey S. Cyclic lipopeptides from Bacillus subtilis activate distinct patterns of defense responses in grapevine. Mol. Plant Pathol., 2015, 16(2): 177-187 (doi: 10.1111/mpp.12170). 22. Kino K., Kotanaka Y., Arai T., Yagasaki M. A novel L-amino acid ligase from Bacillus subtilis NBRC3134, a microorganism producing peptide — antibiotic rhizocticin. Biosci. Biotech. Bioch., 2009, 73(4): 901-907 (doi: 10.1271/bbb.80842). 23. Sharma A. Rhamnolipid producing PGPR and their role in damping off disease suppression. In: Plant bacteria interactions strategies and techniques to promote plant growth /I. Ahmad, J. Pichtel, S. Haya (eds.). Wiley VCH Publications, Weinheim, 2008. 24. Jourdan E., Henry G., Duby F., Dommes J., Barthélemy J.P., Thonart P., Ongena M. Insights into the defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Mol. Plant Microbe In., 2009, 22: 456-468 (doi: 10.1094/MPMI-22-4-0456). 25. Korenblum E., de Araujo L.V., Guimarâes C.R., de Sourza L.M., Sassaki G., Abreu F., Nitschke M., Lins U., Guimarâes-Freire D.M., Barreto-Bergter E., Seldin L. Purification and characterization of a surfactin-like molecule produced by Bacillus sp. H2O-1 and its antagonistic effect against sulfate reducing bacteria. BMC Microbiol., 2012, 12(252): 1-13 (doi: 10.1186/1471- 2180-12-252). 26. McLoon A.L., Guttenplan S.B., Kearns D.B., Kolter R., Losick R. Tracing the domestication of a biofilmforming bacterium. J. Bacteriol., 2011, 193: 2027-2034 (doi: 10.1128/JB.01542-10). 27. Chu F., Kearns D.B., McLoon A., Chai Y., Kolter R., Losick R. A novel regulatory protein governing biofilm formation in Bacillus subtilis. Mol. Microbiol., 2008, 68(5): 1117-1127 (doi: 10.1111/j.1365-2958.2008.06201.x). 28. Chen Y., Yan F., Chai Y., Liu H., Kolter R., Losick R., Guo J. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ. Microbiol., 2013, 15(3): 848-864 (doi: 10.1111/j.1462- 2920.2012.02860.x). 29. Earl A.M., Losick R., Kolter R. Ecology and genomics of Bacillus subtilis. Trends Microbiol., 2008, 16: 269-275 (doi: 10.1016/j.tim.2008.03.004). 30. Branda S.S., Chu F., Kearns D.B., Losick R., Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol., 2005, 59: 1229-1238 (doi: 10.1111/j.1365- 2958.2005.05020.x). 31. Epstein A.K., Pokroy B., Seminara A., Aizenberg J. Bacterial biofilm shows persisten resistance to liquid wetting and gas penetration. PNAS USA, 2011, 108: 995-1000 (doi: 10.1073/pnas.1011033108). 32. Kovács A.T., van Gestel J., Kuipers O.P. The protective layer of biofilm: a repellent function for a new class of amphiphitic proteins. Mol. Microbiol, 2012, 85(1): 8-11 (doi: 10.1111/j.1365- 2958.2012.08101.x). 33. Abdel-Mawgoud A.M., Aboulwafa M.M., Hassouna N.A.-H. Characterization of surfactin produced by Bacillus subtilis isolates BS5. Appl. Biochem. Biotech., 2008, 150(3): 289-303 (doi: 10.1007/s12010-008-8153-z). 34. Leclére V., Béchet M., Adam A., Guez J.-S., Wathelet B., Ongena M., Thonart P., Gancel F., Chollet-Imbert M., Jacques P. Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities mycosubtilin. Appl. Environ. Microb., 2005, 71(8): 4577-4584 (doi: 10.1128/AEM.71.8.4577-4584.2005). 35. Fernandes P.A.V., Arruda I.R., Santos A.F.B., Araujo A.A., Major A.M.S., Ximenes E.A. Antimicrobial activity of surfactants produces by Bacillus subtilis R14 against multidrug-resistant bacteria. Braz. J. Microbiol., 2007, 38: 704-709 (doi: 10.1590/S1517-83822007000400022). 36. Shoeb E., Akhlag F., Badar U., Akhter J., Imtiaz S. Classification and industrial applications of biosurfactants. Academic Research International, 2013, 4(3): 243-252. 37. Debois D., Fernandez O., Franzil L., Jourdan E., de Brogniez A., Willems L., Clément C., Dorey S., De Pauw E., Ongena M. Plant polysaccharides initiate underground crosstalk with bacilli by inducing synthesis of the immunogenic lipopeptide surfactin. FEMS Microbiol. Rev., 2015, 10: 1758-2229 (doi: 10.1111/1758-2229.12286). 38. Cawoy H., Mariutto M., Henry G., Fisher C., Vasilyeva N., Thonart P., Dommes J., Ongena M. Plant defense stimulation by natural isolates of Bacillus depends on efficient surfactin production. Mol. Plant Microbe In., 2014, 27: 87-100 (doi: 10.1094/MPMI-09-13-0262-R). 39. Mohammadipour M., Mousivand M., Jouzani G.S., Abbasalizadeh S. Molecular and biochemical characterization of Iranian surfactin-producing Bacillus subtilis isolates and evaluation of their biocontrol potential against Aspergillus flavus and Colletotrichum gloeosporioides. Can. J. Microbiol., 2009, 55: 395-404 (doi: 10.1139/w08-141). 40. Li J., Yang Q., Zhao L., Zhang S., Wang Y., Zhao X. Purification and characterization of a novel antifungal protein from Bacillus subtilis strain B29. J. Zhejiang Univ.-Sc. B, 2009, 10(4): 264-272 (doi: 10.1631/jzus.B0820341). 41. López D., Vlamakis H., Losick R., Kolter R. Cannibalism enhances biofilm development in Bacillus subtilis. Mol. Microbiol., 2009, 74: 609-618 (doi: 10.1111/j.1365-2958.2009.06882.x). 42. Zhong J., Zhang X., Ren Y., Yang J., Tan H., Zhou J. Optimization of Bacillus subtilis cell growth effecting jiean-peptide production in fed batch fermentation using central composite design. Electron. J. Biotechn., 2014, 17: 132-136 (doi: 10.1016/j.ejbt.2014.04.010). 43. Zhang X., Zhou J., Fu W., Li Z., Zhong J., Yang J., Xiao L., Tan H. Response surface methodology used for statistical optimization of jiean-peptide production by Bacillus subtilis. Electron. J. Biotechn., 2010, 15: 0717-3458 (doi: 10.2225/vol13-issue4-fulltext-5). 44. Meena K.R., Kanwar S.S. Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics. BioMed Res. Int., 2014, 2015: 9 (doi: 10.1155/2015/473050). 45. Willey J.M., van der Donk W.A. Lantibiotics: peptides of diverse structure and function. Annu. Rev. Microbiol., 2007, 61: 477-501 (doi: 10.1146/annurev.micro.61.080706.093501). 46. Hu L.B., Shi Z.Q., Zhang T., Yang Z.M. Fengycin antibiotics isolated from B-FS 01 culture inhibit the growth of Fusarium moniliforme Sheldon ATCC38932. FEMS Microbiol. Lett., 2007, 272: 91-98 (doi: 10.1111/j.1574-6968.2007.00743.x). 47. Sachdev D.P., Cameota S.S. Biosurfactants in agriculture. Appl. Microbiol. Biot., 2013, 97: 1005-1016 (doi: 10.1007/s00253-012-4641-8). 48. Choudhary D.K., Johri B.N. Interactions of Bacillus spp. and plants — with special reference to induced systemic resistance (ISR). Microbiol. Res., 2008, 164: 493-513 (doi: 10.1016/j.micres.2008.08.007). 49. Degering C., Eggert T., Puls M., Bongaerts J., Evers S., Maurer K.-H., Jaeger K. Optimization of protease secretion in Bacillus subtilis and Bacillus licheniformis by screening of homologous and heterologous signal peptides. Appl. Environ. Microb., 2010, 76: 6370-6376 (doi: 10.1128/AEM.01146-10). 50. Borisova S.A., Circello B.T., Zhang J.K., van der Donk W.A., Metcalf W.W. Biosynthesis of rhizocticins, antifungal phosphonate oligopeptides produced by Bacillus subtilis ATCC6633. Chemistry Biology, 2010, 17: 28-37 (doi: 10.1016/j.chembiol.2009.11.017). 51. Parisot J., Carey S., Breukink E., Chan W.C., Narbad A. Molecular mechanism of target recognition by subtilin, a class I lanthionine antibiotic. Antimicrob. Agents Chemother., 2008, 52: 612-618 (doi: 10.1128/AAC.00836-07). 52. Al-Bahry S.N., Al-Wahaibi Y.M., Elshafie A.E., Al-Bemani A.S., Joshi S.J., Al-Makhmari H.S., Al-Sulaimani H.S. Biosurfactant production by Bacillus subtilis B20 using date molasses and its possible application in enhanced oil recovery. Int. Biodeter. Biodegr., 2013, 81: 141-146 (doi: 10.1016/j.ibiod.2012.01.006). 53. Fickers P. Antibiotic compounds from so Bacillus: why are they amazing? American Journal of Biochemistry and Biotechnology, 2012, 8(1): 38-43 (doi: 10.3844/ajbbsp.2012.38.43). 54. Chebotar’ V.K., Makarova N.M., Shaposhnikov A.I., Kravchenko L.V. Antifungal and phytostimulating characteristics of Bacillus subtilis Ch-13 rhizospheric strain, producer biopreparations. Appl. Biochem. Microbiol., 2009, 45(4): 419-423 (doi: 10.1134/s0003683809040127). 55. van Loon L.C. Plant responses to plant growth promoting rhizobacteria. Eur. J. Plant Pathol., 2007, 119: 243-254 (doi: 10.1007/s10658-007-9165-1). 56. Quardros C.P., Teixeira Duarte M.C., Pastore G.M. Biological activities of a mixture of biosurfactant from Bacillus subtilis and alkaline lipase from Fusarium oxysporum. Braz. J. Microbiol., 2011, 42: 354-361 (doi: 10.1590/s1517-83822011000100045). 57. Kino K., Kotanaka Y., Arai T., Yagasaki M. A novel L-amino acid ligase from Bacillus subtilis NBRC3134, a microorganism producing peptide-antibiotic rhizocticin. Biosci. Biotech. Bioch., 2009, 73(4): 901-907 (doi: 10.1271/bbb.80842). 58. Deleu M., Paquot M., Nylander T. Fengycin interaction with lipid monolayers at the airaqueous interface — implications for the effect of fengycin on biological membranes. J. Colloid Interf. Sci., 2005, 283: 358-365 (doi: 10.1016/j.jcis.2004.09.036). 59. Linares J.F., Gustafsson I., Baquero F., Martinez J.L. Antibiotics as intermicrobial signaling agents instead of weapons. PNAS USA, 2006, 103: 19484-19489 (doi: 10.1073/pnas.0608949103). 60. Hamdache A., Lamarti A., Aleu J., Collado I.G. Non-peptide metabolites from the genus Bacillus. J. Nat. Prod., 2011, 74(4): 893-899 (doi: 10.1021/np100853e). 61. Alaa R.K., alden Sanaa B. Antimicrobial effect of phospholipid produced from Bacillus subtilis. World Journal of Experimental Biosciences, 2014, 2(2): 59-63. 62. Kudryashova E.B., Vinokurova N.G., Ariskina E.V. Bacillus subtilis and phenotypically similar strains producing hexaene antibiotics. Appl. Biochem. Microbiol., 2005, 41(5): 486-489 (doi: 10.1007/s10438-005-0087-4). 63. Aloni R., Aloni E., Langhans M., Ullrich C.I. Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Annals of Botany, 2006, 97: 883-893 (doi: 10.1093/aob/mcl027). 64. Yarullina L.G., Kasimova R.I., Ibragimov R.I., Akhatova A.R., Umarov I.A., Maksimov I.V. Qualitative and quantitative changes of potato tuber proteome under the influence of signal molecules and infection with Phytophthora infestans. Appl. Biochem. Microbiol., 2016, 52(1): 71- 78 (doi: 10.1134/S0003683816010154). 65. Vandeputte O., Öden S., Mol A., Vereecke D., Goethals K., El Jaziri M., Prinsen E. Biosynthesis of auxin by the Gram-positive phytopathogen Rhodococcus faseians is controlled by compounds specific to infected plant tissues. Appl. Environ. Microb., 2005, 71(3): 1169-1177 (doi: 10.1128/AEM.71.3.1169-1177.2005). 66. Gordillo A., Maldonado M.C. Purification of peptides from Bacillus strains with biological activity. Chromatography and Its Applications, 2012, 11: 201-225. 67. Dyakov Yu.T., Ozeretskovskaya O.L. Vertical pathosystem: Resistance genes and their products. Immune response. In: Comprehensive and molecular phytopathology /Yu.T. Dyakov, V.G. Dzhavakhiya, T. Korpela (eds.). Elsevier, Amsterdam, 2007: 181-215 (doi: 10.1016/B978-044452132-3/50011-0). 68. Ongena M., Jourdan E., Adam A., Paquot M., Brans A., Joris B., Arpigny J.L., Thonart P. 37 Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol., 2007, 9: 1084-1090 (doi: 10.1111/j.1462-2920.2006.01202.x). 69. Jordan E., Henry G., Duby F., Dommes J., Barthélemy J.P., Thonart P., Ongena M. Insights into defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Mol. Plant Microbe In., 2009, 22: 456-468 (doi: 10.1094/MPMI-22-4-0456). 1 2 |
