Получение и анализ качества лекарственных препаратов производных тиазина
 Скачать 1.13 Mb. Скачать 1.13 Mb.
|
|
Heavy metals (2.4.8) Maximum 10 ppm. 1.0 g complies with test C. Prepare the reference solution using 1 mL of lead standardsolution (10 ppm Pb) R. Loss on drying (2.2.32) Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C. Sulfated ash (2.4.14) Maximum 0.1 per cent, determined on 1.0 g. ASSAY Dissolve 0.250 g in a mixture of 5.0 mL of 0.1 M hydrochloric acid and 50 mL of ethanol (96per cent) R. Carry out a potentiometric titration (2.2.20), using 0.1 M sodium hydroxide. Read the volume added between the 2 points of inflexion. 1 mL of 0.1 M sodium hydroxide is equivalent to 35.53 mg of C17H20Cl2N2S. STORAGE In an airtight container, protected from light. IMPURITIES Specified impurities A, B, C, D, E. 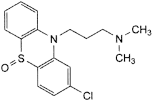 A. 3-(2-chloro-10H-phenothiazin-10-yl)-N,N-dimethylpropan-1-amine S-oxide (chlorpromazine sulfoxide), 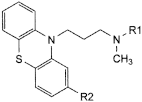 B. R1 = [CH2]3-N(CH3)2, R2 = Cl: N-[3-(2-chloro-10H-phenothiazin-10-yl)propyl]-N,N,N-trimethylpropane-1,3-diamine, C. R1 = CH3, R2 = H: 3-(10H-phenothiazin-10-yl)-N,N-dimethylpropan-1-amine (promazine), D. R1 = H, R2 = Cl: 3-(2-chloro-10H-phenothiazin-10-yl)-N-methylpropan-1-amine (desmethylchlorpromazine), 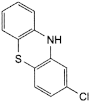 E. 2-chloro-10H-phenothiazine. Chlorpromazine Injection Action and use Dopamine receptor antagonist; neuroleptic. DEFINITION Chlorpromazine Injection is a sterile solution of Chlorpromazine Hydrochloride in Water for Injections free from dissolved air. The injection complies with the requirements stated under Parenteral Preparations and with the following requirements. Content of chlorpromazine hydrochloride, C17H19ClN2S,HCl 95.0 to 105.0% of the stated amount. CHARACTERISTICS A colourless or almost colourless solution. IDENTIFICATION A. To a volume containing 0.1 g of Chlorpromazine Hydrochloride add 20 mL of water and 2 mL of 10M sodium hydroxide. Shake and extract with 25 mL of ether . Wash the ether layer with two 5 mL quantities of water, dry with anhydrous sodium sulfate, evaporate the ether and dissolve the residue in 1 mL of chloroform. The infrared absorption spectrum of the resulting solution, Appendix II A, is concordant with the reference spectrum of chlorpromazine (RS 056). B. Complies with the test for identification of phenothiazines, Appendix III A. For solution (1) dilute the injection with water to give a solution containing 0.2% w/v of Chlorpromazine Hydrochloride. TESTS_Acidity_pH,_5.0_to_6.5,_Appendix_V_L._Related_substances'>TESTS Acidity pH, 5.0 to 6.5, Appendix V L. Related substances Carry out the test for related substances in phenothiazines, Appendix III A, using mobile phase A and applying separately to the plate 20 µL of each of the following freshly prepared solutions. For solution (1) dilute a volume of the injection, if necessary, with sufficient of a mixture of 95 volumes of methanol and 5 volumes of diethylamine to produce a solution containing 0.5% w/v of Chlorpromazine Hydrochloride. For solution (2) dilute 1 volume of solution (1) to 20 volumes with the same solvent. For solution (3) dilute 1 volume of solution (1) to 200 volumes with the same solvent. Any secondary spot in the chromatogram obtained with solution (1) is not more intense than the spot in the chromatogram obtained with solution (2) and not more than one such spot is more intense than the spot in the chromatogram obtained with solution (3). ASSAY Carry out the following procedure protected from light. Dilute a suitable volume with sufficient 0.1M hydrochloric acid to produce a solution containing 0.0005% w/v of Chlorpromazine Hydrochloride and measure the absorbance at the maximum at 254 nm, Appendix II B. Calculate the content of C17H19ClN2S,HCl taking 915 as the value of A(1%, 1 cm) at the maximum at 254 nm. STORAGE_Chlorpromazine_Injection_should_be_protected_from_light._Chlorpromazine_Oral_Solution_Chlorpromazine_Elixir_Action_and_use'>STORAGE Chlorpromazine Injection should be protected from light. Chlorpromazine Oral Solution Chlorpromazine Elixir Action and use Dopamine receptor antagonist; neuroleptic. DEFINITION Chlorpromazine Oral Solution is a solution of Chlorpromazine Hydrochloride in a suitable flavoured vehicle. The oral solution complies with the requirements stated under Oral Liquids and with the following requirements. Content of chlorpromazine hydrochloride, C17H19ClN2S,HCl 90.0 to 110.0% of the stated amount. IDENTIFICATION Carry out the method for identification of phenothiazines, Appendix III A. For solution (1) dilute a suitable volume of the oral solution with water to give a solution containing 0.2% w/v of Chlorpromazine Hydrochloride. TESTS Related substances Carry out the procedure protected from light under an atmosphere of nitrogen. Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions. (1) Add 40 mL of water and 5 mL of a 20% w/v solution of sodium hydroxide to a quantity of the oral solution containing 20 mg of Chlorpromazine Hydrochloride in a separating funnel and swirl to mix. Extract with two 25-mL quantities of chloroform, combine the chloroform extracts and filter through anhydrous sodium sulfate. Wash the sodium sulfate with a further 25 mL of chloroform and evaporate the combined filtrate and washings to dryness at about 30° in a gentle current of nitrogen. Dissolve the residue in 2 mL of a mixture of 5 volumes of diethylamine and 95 volumes of methanol . (2) Dilute 1 volume of solution (1) to 200 volumes with a mixture of 5 volumes of diethylamine and 95 volumes of methanol . (3) 0.030% w/v solution of chlorpromazine sulfoxide BPCRS in a mixture of 5 volumes of diethylamine and 95 volumes of methanol . CHROMATOGRAPHIC CONDITIONS (a) Use as the coating silica gel F254. (b) Use the mobile phase as described below. Use an atmosphere of nitrogen. (c) Apply 10 µL of each solution. (d) Develop the plate to 12 cm. (e) After removal of the plate, dry in air, spray with 20% w/w solution of perchloric acid and heat at 100° for 5 minutes. MOBILE PHASE 10 volumes of acetone, 10 volumes of diethylamine and 80 volumes of cyclohexane. LIMITS In the chromatogram obtained with solution (1): any spot corresponding to chlorpromazine sulfoxide is not more intense than the spot in the chromatogram obtained with solution (3); any other secondary spot is not more intense than the spot in the chromatogram obtained with solution (2). Disregard any spot remaining on the line of application. ASSAY Carry out the following procedure protected from light. Dilute a quantity containing 0.1 g of Chlorpromazine Hydrochloride to 500 mL with 2M hydrochloric acid . To 10 mL of this solution add 20 mL of water, make distinctly alkaline to litmus paper with 13.5M ammonia and extract with six 25 mL quantities of ether . Extract the combined ether solutions with four 25 mL quantities of a mixture containing 1 volume of hydrochloric acid and 99 volumes of water, discard the ether, remove any dissolved ether from the combined extracts with a current of air and dilute to 250 mL with a mixture containing 1 volume of hydrochloric acid and 99 volumes of water. Measure the absorbance of the resulting solution at the maximum at 254 nm, Appendix II B. Calculate the content of C17H19ClN2S,HCl taking 914 as the value of A(1%, 1 cm) at the maximum at 254 nm. STORAGE Chlorpromazine Oral Solution should be protected from light. Chlorpromazine Tablets Action and use Dopamine receptor antagonist; neuroleptic. DEFINITION Chlorpromazine Tablets contain Chlorpromazine Hydrochloride. They are coated. The tablets comply with the requirements stated under Tablets and with the following requirements. Content of chlorpromazine hydrochloride, C17H19ClN2S,HCl 92.5 to 107.5% of the stated amount. IDENTIFICATION A. To a quantity of the powdered tablets containing 40 mg of Chlorpromazine Hydrochloride add 10 mL of water and 2 mL of 10M sodium hydroxide. Shake and extract with 15 mL of ether . Wash the ether layer with two 5-mL quantities of water, dry with anhydrous sodium sulfate and evaporate the ether. Dissolve the residue in 0.4 mL of chloroform. The infrared absorption spectrum of the resulting solution, Appendix II A, is concordant with the reference spectrum of chlorpromazine (RS 056). B. Comply with the test for identification of phenothiazines, Appendix III A. For solution (1) shake a quantity of the powdered tablets with sufficient chloroform to produce a solution containing 0.20% w/v of Chlorpromazine Hydrochloride, centrifuge and use the supernatant liquid. TESTS Related substances Comply with the test for related substances in phenothiazines, Appendix III A, using mobile phase A and the following freshly prepared solutions. For solution (1) extract a quantity of the powdered tablets containing 0.1 g of Chlorpromazine Hydrochloride with 10 mL of a mixture of 95 volumes of methanol and 5 volumes of diethylamine and filter. For solution (2) dilute 1 volume of solution (1) to 200 volumes with the same solvent mixture. Dissolution Carry out the procedure protected from light. Comply with the requirements for Monographs of the British Pharmacopoeia in the dissolution test for tablets and capsules, Appendix XII B1. TEST CONDITIONS (a) Use Apparatus 2, rotating the paddle at 50 revolutions per minute. (b) Use 900 mL of 0.1M hydrochloric acid , at a temperature of 37°, as the medium. PROCEDURE (1) After 45 minutes withdraw a 10 mL sample of the medium and measure the absorbance of the filtered sample, suitably diluted with the dissolution medium to produce a solution containing 0.0005% % w/v of Chlorpromazine Hydrochloride, at the maximum at 254 nm, Appendix II B using 0.1M hydrochloric acid in the reference cell. DETERMINATION OF CONTENT Calculate the total content of C17H19ClN2S,HCl, in the medium taking 914 as the value of A(1%, 1 cm) at the maximum at 254 nm. ASSAY Carry out the following procedure protected from light. Powder 10 tablets without loss, triturate the powder with 10 mL of absolute ethanol , add about 300 mL of 0.1M hydrochloric acid and shake for 15 minutes. Add sufficient 0.1M hydrochloric acid to produce 500 mL, filter, dilute a volume of the filtrate containing 5 mg of Chlorpromazine Hydrochloride to 100 mL with 0.1M hydrochloric acid and further dilute 10 mL to 100 mL with the same solvent. Measure the absorbance of the resulting solution at the maximum at 254 nm, Appendix II B. Calculate the content of C17H19ClN2S,HCl taking 915 as the value of A(1%, 1 cm) at the maximum at 254 nm. |
