Моль. Вещество это любая совокупности атомов и молекул, находящаяся в определенном агрегатном состоянии. Атом
 Скачать 190.34 Kb. Скачать 190.34 Kb.
|
|
I. 8. Kimyoviy reaksiyalarlarning turlari Birikish reaksiyalari Oddiy moddalarning o’zaro birikishi Na2O, CaS, CO, Na2S, CO2, CuCl2, P2O3, ZnCl2, SO2, CaCl2, CaO, NH3, HCl, HJ, HBr, H2S, HF, SiO2, MgO, SnCl2, P2O5, AlCl3, Al2O3, CuO, FeCl3, K2O, NaCl, CuS, Cr2S3; Na+O2→ Na2O K+O2→ CII+O2→CuII+O2→ CIV+O2→ Al+O2→ PIII+O2→ PV+O2→ SIV+O2→ Mg+O2→ Ca+O2→ SiIV+O2→ H2+Cl2→H2+S→ H2+Br2→ H2+I2→ H2+F2→ H2+N2→ SnII+Cl2→ Ca+Cl2→ Al+Cl2→ Zn+Cl2→ FeIII+Cl2→ CuII+Cl2→ Na+Cl2→ Na+S→ CrIII+S→ Ca+S→ Cu+S→ Asosli oksidlarga suvning ta’siri Na2O + H2O → NaOH K2O + H2O → Rb2O + H2O → MgO + H2O → CaO + H2O → BaO + H2O → Kislotali oksidlarga suvning ta’siri H3BO3, HBrO4, HNO2, HBrO3, N2O5, H3AsO4, H3PO4, HClO3, H2SO4, HClO, HClO2, H2SO3, HClO4, H3PO3, HBrO, HNO2+HNO3, HBrO3, H2CO3; B2O3 + H2O→ H3BO3CO2+ H2O→ N2O3+ H2O→ NO2+ H2O→ N2O5+ H2O →P2O3+ H2O→ P2O5+ H2O→SO2+ H2O→ SO3+ H2O→Cl2O + H2O→ Cl2O3+ H2O→Cl2O5+ H2O→ Cl2O7+ H2O→As2O3+ H2O→ Br2O + H2O→Br2O3+ H2O→ Br2O5+ H2O→Br2O7+ H2O→ Asosli oksidlarga kislotali oksidlarning ta’siri Na3BO3, K3PO4, NaNO2, NaNO3, Ba3(PO3)2, CaCO3, CaSO3, Mg(BrO4)2, NaClO, NaBrO3, KClO3, KBrO, Ca3(AsO4)2, Mg(ClO4)2, Ba(BrO2)2, RbClO2, Na2SO4; B2O3 + Na2O → Na3BO3CO2+ CaO →N2O3+ K2O →P2O3+ BaO →P2O5+ K2O →SO2+ CaO →SO3+ Na2O →Cl2O + Na2O →Cl2O3+ Rb2O →Cl2O5+ K2O →Cl2O7+ MgO →As2O3+ CaO →Br2O + K2O →Br2O3+ BaO →Br2O5+ Na2O →Br2O7+ MgO → N2O5+ Na2O → O’rin olish reaksiyalari Metal oksidlariga vodorodning ta’siri FeO+H2→ Fe +H2O; CuO+H2→ Fe2O3+H2→ CrO+H2→ Cr2O3+H2→ZnO+H2→ SnO+H2→WO3+H2→ Metal oksidlariga is gazining ta’siri FeO+CO→ Fe +CO2; CuO+CO→ Fe2O3+CO→ CrO+CO→ Cr2O3+CO→ZnO+CO→ SnO+CO→WO3+CO→ Metallarga kislotalarning ta’sri Na+HCl→ NaCl +H2; Ca+HCl→ K+HCl→Zn+HCl→ Fe+HCl→Na+H2SO4→ Ca+H2SO4→K+H2SO4→ Zn+H2SO4→Fe+H2SO4→ Na+H2SO3→Ca+H2SO3→ K+H2SO3→Zn+H2SO3→ Fe+H2SO3→ Parchalanish reaksiyalari Tuzlarning parchalanishi NaHCO3→ Na2CO3 + CO2 + H2O KHCO3→ CaCO3→ CaO + CO2 MgCO3→ AgNO3→ Ag + NO2 + O2 Hg(NO3)2→ Cu(NO3)2→ CuO + NO2 + O2 Mg(NO3)2→ NaNO3→ NaNO2+ O2 KNO3→ CaSO3→CaO + SO2 BaSO3→ BeSO3→ Na2SO3→ Asoslarning parchalanishi Fe2O3, Al2O3, FeO, ZnO, CuO, MnO, Cr2O3, CrO; Fe(OH)3→ Fe2O3+ H2O Fe(OH)2→ … + H2O; Cu(OH)2→ … + H2O Cr(OH)3→ … + H2O; Cr(OH)2→ … + H2O Mn(OH)2→ …+ H2O; Zn(OH)2→ … + H2O Al(OH)3→ Kislotalarning parchalanishi SO2, Cr2O3, SiO2, ZnO, CO2, Al2O3; H2SO3→ SO2 + H2O; H2SiO3→…+ H2O H2CO3→…+ H2O; H3AlO3 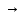 …+ H2O …+ H2OH2ZnO2→…+ H2O; H3CrO3→…+ H2O Almashinish reaksiyalari Asoslar va kislotalarning o’zaro ta’siri NaOH+HCl→ NaCl + H2O KOH+HCl→ Ba(OH)2+HCl→ Mg(OH)2+HCl→ Ca(OH)2+HCl→ Al(OH)3+HCl→ Cu(OH)2+HCl→ Fe(OH)3+HCl→ Fe(OH)2+HCl→ NaOH+H2SO4→ Na2SO4 + H2O KOH+H2SO4→Ba(OH)2+H2SO4→ Mg(OH)2+H2SO4→Ca(OH)2+H2SO4→ Al(OH)3+H2SO4→Cu(OH)2+H2SO4→ Fe(OH)3+H2SO4→Fe(OH)2+H2SO4→ Zn(OH)2+H2SO4→ NaOH+HNO3→ NaNO3 + H2O KOH +HNO3→Ba(OH)2+HNO3→ Mg(OH)2+HNO3→Ca(OH)2+HNO3→ Al(OH)3+HNO3→Cu(OH)2+HNO3→ Fe(OH)3+HNO3→Fe(OH)2+HNO3→ Zn(OH)2+HNO3→ Tuzlarga kislotalarning ta’siri Na2CO3+HCl→ NaCl + CO2 + H2O NaHCO3+HCl→ K2CO3+HCl→ KHCO3+HCl→ CaCO3+HCl→ Ca(HCO3)2+HCl→ MgCO3+HCl→ Mg(HCO3)2+HCl→ Na2CO3+H2SO4→Na2SO4 + H2O NaHCO3+H2SO4→K2CO3+H2SO4→ KHCO3+H2SO4→ CaCO3+H2SO4→ MgCO3+H2SO4→Mg(HCO3)2+H2SO4→ Tuzlarga asoslarning ta’siri CuSO4+KOH→ K2SO4 + Cu(OH)2 FeCl3+KOH→Zn(NO3)2+KOH→ Fe2(SO4)3+KOH→ CrCl2+KOH→ CrCl3+KOH→Al(NO3)3+KOH→ MnSO4+KOH→ CuSO4+NaOH→ FeCl3+NaOH→Zn(NO3)2+NaOH→ Fe2(SO4)3+NaOH→ CrCl2+NaOH→ CrCl3+NaOH→Al(NO3)3+NaOH→ MnSO4+NaOH→ Tuzlarning o’zaro ta’siri NaCl+AgNO3→ NaNO3 + AgCl 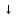 KCl+AgNO3→ BaCl2+AgNO3→ Na2SO4+BaCl2→ K2SO4+Ba(NO3)2→ Na2S+CuSO4→ K2S+Zn(NO3)2→ Tuzlarning gidrolizi(suv bilan ta’siri) Al2S3+H2O→Al(OH)3 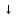 + H2S + H2S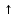 AlN+H2O→ CH3COONH4+H2O→ CaC2+H2O→ Al4C3+H2O→ Cr2S3+H2O→ Reaksiyani davom ettiring (1-132)
I.10. Murkkab moddalarning formulalarini topish I. 1.Tarkibida 11,11%-H, 88,89%-O bo’lgan murakkab moddaning fo’rmulasini toping. A)H2O2B)H2O C)H3O D)D2O Решение: 1) elementlar foizlarini мольekulyar massasiga bo’lib nisbatlar topiladi. H= 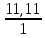 = 11.11 моль, O= = 11.11 моль, O= 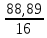 = 5,55 моль = 5,55 моль 2) мольi kichik bo’lganiga bo’lib nisbat topiladi. H = 11,11:5,55 = 2 3)murakkab moddada H=2 va O=1 demak O = 5,55:5,55 = 1 H2O formulaga mos keladi. ОТВЕТ:H2O 2. Kimyoviy birikma tarkibida massa ulushlari quyidagicha H-1,59 %, N-22,22 %, O-76,19 %. Shu birikmaning formulasini toping. A)HNO3 B)HNO2 C)H2NO3 D)HNO Решение: 1) H= 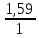 = 1,59 моль, N= = 1,59 моль, N= 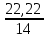 = 1,59 моль, O= = 1,59 моль, O= 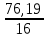 = 4,76 моль = 4,76 моль2) H = 1,59:1,59 = 1 3) formulasi HNO3 N = 1,59:1,59 = 1 O = 4,76:1,59 = 3 Массовые доли кислорода, фосфора и водорода в кислоте составляют соответственно 62,92%, 34,83% и 2,25%. Определите формулу кислоты. 3. Tarkibida 40% oltingugurt va 60% kislorod bo’lgan birikmaning formulasini toping. A)SO2 B)SO C)SO3 D)SO6 4. Tarkibida 30,4% azot va 68,6% kislorod bo’lgan birikmaning formulasini toping. A)NO2 B)NO C)N2O D)N2O3 5. 92,62% simob va 7,38% kisloroddan iborat moddaning oddiy formulasini toping. A)HgO2 B)Hg2O C)HgO D)HgO4 6. Tarkibida 70% tеmir va 30% kislorod bo’lgan birikmaning oddiy formulasini chiqaring. A)Fe2O3 B)FeO C)Fe3O4 D)FeO2 7. 44,82% kaliy, 18,4% oltingugurt va 36,78% kisloroddan iborat birikmaning oddiy formulasini chiqaring. A)K2SO3 B)K2S2O3C)K2SO4 D)KSO3 8. Birikma 13,04% vodorod, 34,78% kislorod va 52,17% uglеroddan tashkil topgan. Shu birikmaning oddiy formulasini chiqaring. A)C2H5OH B)H2CO3 C)CH3OH D)C3H7OH 9. Birikmada 22,13% alyuminiy, 25,41% fosfor va 52,46% kislorod bor. Birikmaning formulasini toping. A)AlPO3 B)Al2(PO3)3C)AlPO4 D)Al4(P2O7)3 10. Birikmada 1,59% vodorod, 22,22% azot, 76,20% kislorod bo’lgan birikmaning haqiqiy formulasini toping. A)HNO3 B)HNO2 C)NH4NO2 D)NH4OH 11. Tarkibida 32,4% natriy, 22,53% oltingugurt va 45,07% kislorod bo’lgan birikmaning haqiqiy formulasini aniqlang. A)Na2SO3 B)Na2S2O3C)Na2SO4 D)NaHSO4 12. Мольеkulyar massasi 85 bo’lgan moddaning elеmеntlar miqdori quyidagicha: 27,06% Na, 16,47% azot, 56,47% kislorod. Birikmaning formulasini toping. A)NaNO2B)NaNO3 C)NaNO D)KNO3 13. Elеmеntlar mikdori: Mg-28,6%, C-14,3%, O-57,14%. Мольеkulyar massasi 84 bo’lgan birikmaning formulasini toping. A)MgCO3 B)(HCOO)2Mg C)Mg(HCO3)2 D)CaCO3 14. Elеmеntlar miqdori: Ca-29,4%, H-0,735%, P-22,8%, O-47,06% bo’lgan birikmaning haqiqiy formulasini toping. A)Ca3(PO4)2 B)Ca(H2PO4)2C)CaHPO4 D)Ca(OH)H2PO4 15. Birikma 52,94%-A1 va 47,06%-O2dan iborat. Birikmaning мольеkulyar formulasini chiqaring. A)Al2O3 B)AlO2 C)AlO D)Al3O4 16. Birikma 2,04% H, 32,65% S va 65,3% O dan iborat.Murakkab moddaning formulasini toping. A)H2SO3B)H2SO4 C)H2S2O3 D)H2S 17. Tarkibida 3,06%-H, 31,63%-P va 65,3%-O bor.Shu moddani formulasini toping. A)H3PO4 B)HPO3 C)H3PO3 D)H4P2O7 18. Birikma 21,21%-N, 6,06%-H, 24,24%-S, 48,48%-O dan iborat.Xaqiqiy formulasini toping. A)(NH4)2SO3B)(NH4)2SO4 C)NH4SO4 D)(NH4)2S2O3 19. Birikmada 38,71%-Ca, 20,0%-P va 41,29%-O dan tashkil topgan. Shu birikmaning formulasini toping. A)Ca3(PO4)2 B)CaPO4 C)Ca(PO3)2 D)Ca3(PO3)2 20. Birikmada 27,48% magniy, 23,66% fosfor va 48,85% kislorod bor. Birikmaning oddiy formulasini chiqaring. A)Mg3(PO4)2 B)MgPO4 C)Mg(PO3)2 D)Mg3(PO3)2 21. Tarkibida 16,08%-Me, 4,2%-C, 6,99%-H, 72,73-O bor birikmaning formulasini toping. A)Na2CO3∙9H2O B)Na2CO3∙10H2O C)Ca(HCO3)2 D)CaCO3∙H2O 22. Tarkibida massa ulushlari quyidabicha 31,84%-Me, 39,18%-O, 28,98%-Cl, bo’lgan modaning fo’rmulasini toping. A)KClO3 B)NaClO2 C)NaClO3 D)LiClO3 |
