Английский. пособие Химики АЯ. Introduction
 Скачать 3.05 Mb. Скачать 3.05 Mb.
|
|
Text 3 Active Vocabulary property (n) – свойство volume (n) – объем appearance (n) – внешний вид application (n) – применение behave (v) – работать density (n) – плотность; концентрация hardness (n) – твёрдость; жёсткость flammability (n) – воспламеняемость corrosion/oxidation resistance (phr) – антикоррозийная стойкость undergo (n) – подвергаться 1. All materials (glass, wood, rubber, steel, etc) have various properties. What words are used to describe these properties? 2. Look at the pictures and describe the properties of these materials.
3. Read the text and choose the correct answer, A, B, C or D for questions 1–5. Properties of Materials A material is any substance or mixture of substances that occupies a volume, and has a mass. A substance usually refers to pure compound. Appearance, behavior, name, structure, color, order, composition and any information about a substance are properties of a material. We most likely recognize a material by its appearance, sometimes aided by other senses such as touch, smell, and taste. Our senses detect some properties of a material, but we rely more on instruments and tests of other properties for an objective identification and analysis. Knowing the properties helps us to develop the ability not only to identify but also to determine the amount and composition of a material. Furthermore, for purposes of management, application, and utilization, we need to know how a material behaves under a set of circumstances. Thus, to help us characterize, recognize, manage, and utilize materials, we need to know its properties. Physical and Chemical Properties of Materials Physical properties are those that can be observed without changing the identity of the substance. The general properties of matter such as color, density, hardness, are examples of physical properties. Properties that describe how a substance changes into a completely different substance are called chemical properties. Flammability and corrosion/oxidation resistance are examples of chemical properties. The difference between a physical and chemical property is straightforward until the phase of the material is considered. When a material changes from a solid to a liquid to a vapor it seems like them become a different substance. However, when a material melts, solidifies, vaporizes, condenses or sublimes, only the state of the substance changes. Consider ice, liquid water, and water vapor, they are all simply H2O. Phase is a physical property of matter and matter can exist in four phases – solid, liquid, gas and plasma. Importance of Properties of Materials for a Chemist The reason properties are so important to a chemist is chemists use them to describe matter and the changes it undergoes. They also use properties to put matter into major categories or classifications. For example, if you have a substance that can be physically separated out into other substances, you have a mixture. A mixture is material made up of two or more substances that are physically mixed but not chemically combined. Take ocean water as an example. The major components of ocean water are water and salt. They can be separated by allowing the water to evaporate. This is a physical separation because we started with salt water, and we ended with salt and water vapor (which is still water). Solutions and alloys are examples of mixtures. If you can't separate it physically, then you have a pure substance. There are only two types of pure substances: elements and compounds. An element is a pure substance made up of only one type of atom. Examples of elements include hydrogen, sodium, neon, gold and copper. A compound is a pure substance that is made up of two or more different elements that are chemically combined. Several common compounds include water (H2O), limestone (CaCO3), salt (NaCl) and carbon dioxide (CO2). It is not easy to separate the elements in a compound because it requires a chemical reaction. For example, if you wanted to separate the oxygen from the hydrogen in water, you would have to do a lot more than just pull them apart. They would have to undergo a chemical reaction to split them. 1. Properties of materials are determined by ……… A an application. B a volume and mass. C a composition and structure. D appearance, behavior, name, structure, color, order, composition. 2. Physical properties are any properties of matter which…….. A measure the potential for undergoing a chemical change. B reveal themselves only when the sample is changed by a chemical reaction. C can be perceived or observed without changing the chemical identity of the sample. D include reactivity, flammability and oxidation states. 3. Examples of chemical properties include………………… A color, molecular weight and volume. B density, hardness. C reactivity, flammability and oxidation states. D plasticity, absorption, emission, ductility. 4. The term……. is used to describe the phase change of a liquid to a gas. A boiling B condensation C melting D freezing 5. Chemists use properties ……………….. A to describe and classify matter. B to make mixtures. C to separate out substances. D to identify materials. 4. The gapped words below all describe physical or chemical properties of substances. The meaning of each word is given on the right. Complete the words with the correct vowels (a, e, i, o, u).
5. Make statements to describe the properties of the following materials. Glass, rubber, steel, wood, polythene, wool, paper, porcelain. 6.Make statements comparing the following materials. 1 glass/fragile/steel 2 paper/flimsy/wood 3 copper/ductile/iron 4 rubber/rigid/steel 5 cardboard/stiff/paper 6 polythene/brittle/material/glass 7 iron/malleable/wood 8 paper/strong/cardboard 9 porcelain/resilient/material/plastic 10 wood/hard/cardboard 11 copper/good/conductor/lead 12 iron/poor/conductor/aluminium 7. Choose the correct word to complete the sentences. It is possible to extraction/ extract/ extracted hydrogen from a widespread source – water. A dye appears coloured because its molecules absorb/ absorbent/ absorption light from a part of the visible spectrum. Pewter is an alloy of usually 80 % tin and 20 % lead. Adding the lead gives the alloy a blueish tinge and increases the malleability/ malleable. In the near-infrared waveband the reflective/ reflect/ reflectivity of water drops to almost zero. New analyses on Antarctic samples have found no detect/ detectable/ detection iridium imprint above background due to cosmic dust. 8. Fill in the appropriate word from the list below into the text and then translate the sentences into Russian: new, change, element, matter, chemical, physical, vapor. There are four main states of ___1): solids, liquids, gases and plasmas. Each of these states is also known as a phase. Elements and compounds can move from one phase to another phase when special physical forces are present. One example of those forces is temperature. The phase or state of matter can ___2) when the temperature changes. Generally, as the temperature rises, matter moves to a more active state. Phase describes a physical state of matter. The key word to notice is physical. Things only move from one phase to another by physical means. If energy is added (like increasing the temperature or increasing pressure) or if energy is taken away (like freezing something or decreasing pressure) you have created a ___3) change. One compound or ___4) can move from phase to phase, but still be the same substance. You can see water ___5) over a boiling pot of water. That vapor (or gas) can condense and become a drop of water. If you put that drop in the freezer, it would become a solid. No matter what phase it was in, it was always water. It always had the same ___6) properties. On the other hand, a chemical change would change the way the water acted, eventually making it not water, but something completely ___7). 9. In pairs, answer the questions. 1. Which is more reactive, aluminium or gold? 2. Which is more brittle, glass or steel? 3. Which is more luminescent, copper or neon? 4. Which is more dense, hydrogen or oxygen? 5. Which is more flammable, ethanol or water? 6. Which is more viscous, blood or water? 10. Think of three different materials you often use in your research. Explain to a partner the physical or chemical properties of the materials and how you use them in your research. Text 4 Active Vocabulary reaction rate (phr) – скорость реакции affect (v) – влиять influence (v) – влиять increase (v) – усиливать course of a reaction (phr) – течение реакции catalyst (n) – катализатор forward reaction (phr) – прямая реакция reverse reactions (phr) – обратная реакция speed up (v) – увеличивать скорость, ускорять reactant (n) – взаимодействующее вещество, реагирующее вещество, реагент participate in (v) – участвовать accelerate (v) – увеличивать скорость shift (v) – перемещать, смещать, сдвигать 1. Copy out this diagram, completing the spaces to show the relationship between solids, liquids and gases, and elements, compounds and mixtures.
The diagram illustrates the relationship between solids, liquids and gases, and compounds, mixtures and elements. The basic constituents of all matter are e________. Some of these exist freely in nature (carbon, iron, copper, etc.). When two or more e_________ are chemically joined together, they form a c____________. For example, oxygen + hydrogen® water. When two or more e________ or c__________ are mixed together but not chemically combined, they form a m__________. E___________, c_________ and m___________ can be either solids, liquids or gases. 2. Look at the description of an atom and the diagram. An atom is defined as the smallest part into which an element may be split without losing its identity as part of the element from which it is divided. Now make statements about the atom, using the following notes. atom/ consist/ nucleus/ electrons nucleus/ consist/ protons/ neutrons proton/ positive charge neutron/ no electrical charge mass/proton/ equal to/ mass/ neutron electron/ negative electrical charge/ negligible mass when/ number/ electrons/ equal to/ number/ protons/ atom/ neutral when/ atom/ gain/ electron/ become/ negative/ ion when/ atom/ lose/ electron/ become/ positive/ ion atomic weight/ atom/ equal to/ number/ protons/ plus/ number/ neutrons atomic number/ atom/ equal to/ protons/ in/ atom 3. Elements and compounds react with each other in numerous ways. What main types of chemical reactions do you know? What conditions are important to the speed of the reaction? Can any conditions be altering during the reaction? Read the text and find out. Reaction Rate The reaction rate for a product in a particular reaction is defined as the amount (in moles or mass units) per unit time per unit volume that is formed or removed. Knowledge of these rates is essential in chemical engineering and environmental engineering. There are several factors that affect the rate of reaction: Temperature: Conducting a reaction at a higher temperature puts more energy into the system and increases the reaction rate. The influence of temperature is described by the Arrhenius equation, whose result is factored into the equation by k. As a rule of the thumb, the reaction rate doubles for every 10 degrees Celsius increase in temperature. Concentration: As reactant concentration increases, the frequency of collision increases and so therefore does the frequency of collisions having sufficient energy to cause reaction. Pressure: The rate of gaseous reactions usually increases with an increase in pressure. Increase in pressure in fact is equivalent to an increase in concentration of the gas. Light: Light is a form of energy. It may affect the rate or even course of a reaction. For example when methane reacts with chlorine in dark, the reaction rate would be very low. It can be speeded up when the mixture is put under diffused light. In bright sunlight, the reaction is explosive. Order: Clearly the order of the reaction has a major effect on its rate. The order of a reaction is found experimentally, and, for most basic reactions, is an integer value. A catalyst: The presence of a catalyst increases the reaction rate in both the forward and reverse reactions by lowering the activation energy of the reaction. State of sub-division of reactants: The larger the surface area compared to the volume, the faster a reaction can take place, as more simultaneous reactions can occur. The nature of the reactants: If a reaction involves the breaking and reforming of bonds (complex) compared to just the forming of bonds (simple) then it generally takes longer. The reactants position in the reactivity series also affects reaction rate. Catalyst A catalyst (Greek: καταλύτης, catalytis) is a substance that accelerates the rate of a chemical reaction, at some temperature, but without itself being transformed or consumed by the reaction (see also catalysis). A catalyst participates in the reaction but is neither a chemical reactant nor a chemical product. In some rare situations, one may describe an atomic nucleus as a catalyst in a nuclear reaction (see, for example, the CNO cycle). More generally, one may sometimes call anything which accelerates a reaction without itself being consumed or transformed a catalyst. This article will focus on chemical catalysts. Catalysts enable reactions to occur much faster or at lower temperatures because of changes that they induce in the reactants. Catalysts provide an alternative pathway, with a lower activation energy, for a reaction to proceed. This means that catalysts reduce the amount of energy needed to start a chemical reaction. Molecules that would not have had the energy to react or that have such low energies that they probably would have taken a long time to react are able to react in the presence of a catalyst. Thus, more molecules that need to gain less energy to react will go through the chemical reaction. The two main categories of catalysts are heterogeneous and homogeneous catalysts. Heterogeneous catalysts are present in different phases from the reactants in the reaction they are catalysing, whereas homogenous catalysts are in the same phase. A simple model for heterogeneous catalysis involves the catalyst providing a surface on which the reactants (or substrates) temporarily become adsorbed. Bonds in the substrate become weakened sufficiently for new products to be created. The bonds between the products and the catalyst are weaker, so the products are released. Homogenous catalysts generally react with one or more reactants to form a chemical intermediate that subsequently reacts to form the final reaction product, in the process regenerating the catalyst. The following is a typical catalytic reaction scheme, where C represents the catalyst: A + C → AC (1) B + AC → AB + C (2) Although the catalyst (C) is consumed by reaction 1, it is subsequently produced by reaction 2, so for the overall reaction: A + B + C → AB + C the catalyst is neither consumed nor produced. Enzymes are biocatalysts. Use of "catalyst" in a broader cultural sense is in rough analogy to the sense described here. Some of the most famous catalysts ever developed are the Ziegler-Natta catalysts used to mass produce polyethylene and polypropylene. Probably the best-known catalytic reaction is the Haber process for ammonia synthesis, where ordinary iron is used as a catalyst. Catalytic converters break down some of the nastier byproducts of automobile exhaust. They are made from platinum and rhodium. Although catalysts will change rate of a reaction, they will not shift an equilibrium reaction. This is because a catalyst affects the rate of reaction equally in both the forward and reverse directions. 4. Answer the questions. What factors affect the rate of reaction? Describe them. What forms are preferable for chemical reactions? What is a catalyst? What types of catalysts do you know? Are enzymes catalysts? Why don’t catalysts shift an equilibrium reaction? 5 (a). Give Russian equivalents for the following English words, word combinations and chemical terms. 1) particular reaction; 2) amount per unit time per unit volume; 3) essential; 4) equation; 5) reaction rate doubles; 6) frequency of collision; 7) sufficient energy; 8) to cause reaction; 9) reacts in dark; 10) diffused light; 11) explosive; 12) major effect; 13) basic reactions; 14) integer value; 15) by lowering the activation energy of the reaction; 16) simultaneous reactions; 17) occur; 18) breaking and reforming of bonds (complex); 19) reactivity series. 5 (b). Give English equivalents for the following Russian words, word combinations and chemical terms. 1) количество; 2) проводить; 3) закон Аррениуса; 4) разлагать на множители; 5) приближённый подсчет; 6) вызывать реакцию; 7) взрывчатый; 8) присутствие катализатора; 9) площадь поверхности; 10) происходить; 11) включать в себя; 12) сосредоточивать; 13) потреблять, расходовать; 14) делать возможным, давать возможность; 15) увеличивать, усиливать, расти; 16) освобождать, выпускать, выделять; 17) схема реакции; 18) суммарная (итоговая, полная) реакция; 19) конвертер, преобразователь; 20) побочные продукты. 6. Complete the C-test. Supply the missing 25 word parts. Reaction Velocity In many cases heat plays an important role, either starting or accelerating a chemical reaction. In general heat is important under conditions where the reac__ __ __ __1) substances are all in the gaseous st__ __ __2), or where they are all in the liquid state or are all in solution. In order that atoms and mole__ __ __ __ __3) should combine and form comp__ __ __ __ __4) they must approach so near each other that their attractive for__ __ __5) are great enough to hold them together in compounds. Having heated up two reacting gases, we sp__ __ __6) up the atoms and molecules; their force of act__ __ __7) becomes greater and their union in compounds takes place or is greatly accel__ __ __ __ __ __8). It probably is generally kn__ __ __9) that together indefinitely at atmospheric pres__ __ __ __10) without reacting; when, however, their temperature is increased, they will combine forming a compound. Substances in solu__ __ __ __11) or in liquid form present an ideal case for chemical reactions. The vol__ __ __12) is very compact as compared to the gas__ __ __ __13) state, and yet the molecules are free to mo__ __14). They may come easily in con__ __ __ __15) with each other and combine. In such cases, having increased the tempe__ __ __ __ __ __16), we greatly accelerate the reac__ __ __ __17), speeding up the motion of the molecules so that they appr__ __ __ __18) each other with great forces. Lowe__ __ __ __19) the temperature, we sl__ __20) down the molecular motion, and the speed of reaction is decr__ __ __ __ __21) or entirely stopped. On the other hand, when a gas__ __ __ __22) substance is diss__ __ __ __ __23) in a solution, any chemical reaction that might oc__ __ __24) between the gas and the solu__ __ __ __25) can be slowed down by increasing the temperature. 7. Match the verbs (1–7) to their meanings (a-g). Make up sentences with the verbs.
8. Complete the paragraph describing the procedure by putting the verb in brackets into the correct form. The surface (1)______ (remove) from 0.99g potassium so that no potassium oxide (2)___ (remain). The potassium (3)_______ (mix) with 0.062 g of iron dendrimer catalyst, 12 ml benzene, 2.7 ml tetrachloroethylene. The mixture was stirred for one week at room temperature. The potassium (4)______ (remove) and washed with t-butyl alcohol, then methanol, and finally water. The reaction product______ (5) (isolate) by centrifugation and decantation of the liquid. Transmission electron microscopy (6)_______(show) the presence of CNTs in the reaction product. The tubes had diameters around 15–20 nm. 9. Give English translation for: Скорость химической реакции – это величина, показывающая как изменяются концентрации исходных веществ или продуктов реакции за единицу времени. Скорость химической реакции зависит от природы реагирующих веществ и условий протекания реакции: концентрации, температуры, а также от давления – для газовых реакций, от измельчения – для твердых веществ, от радиоактивного облучения). Катализаторы – это вещества, которые повышают скорость химической реакции. Они вступают во взаимодействие с реагентами с образованием промежуточного химического соединения и освобождаются в конце реакции. Влияние, оказываемое катализаторами на химические реакции, называется катализом. По агрегатному состоянию, в котором находятся катализатор и реагирующие вещества, следует различать: гомогенный катализ (катализатор образует с реагирующими веществами гомогенную систему, например, газовую смесь; гетерогенный катализ (катализатор и реагирующие вещества находятся в разных фазах; катализ идет на поверхности раздела фаз). 10. Write down a chemical equation. Describe the chemical reaction. 11. Write a report on the topic: ‘Factors affecting chemical reaction rates’. Unit 3. Experimental Chemistry Text 1 Active Vocabulary experiment (n) – опыт, эксперимент; проба verify (v) – проверять, контролировать, подтверждать falsify (v) – опровергать; доказывать ложность perform (v) – исполнять, выполнять; делать, совершать measurement (n) – измерение refer to (v) – относить, приписывать capture (v) – завладеть, захватить, увлечь apply to (v) – относиться; применяться; распространяться tend to (v) – стремиться elimination (n) – устранение valid (adj) – эффективный, надёжный, действенный trial (n) – испытание, проба empirical approach (phr) – эмпирический, опытный подход 1. Read the statements and say whether you agree or disagree with them. “We have to learn again that science without contact with experiments is an enterprise which is likely to go completely astray into imaginary conjecture” – Hannes Alfven (Swedish scientist, physicist). “Today's scientists have substituted mathematics for experiments, and they wander off through equation after equation, and eventually build a structure which has no relation to reality” – Nikola Tesla (Serbian-American inventor, physicist). 2. In pairs, discuss the following questions. 1. What important discoveries have chemists made? Think about medicine, vaccines and industry. 2. What practical uses have these discoveries had? 3. Are chemical experiments interesting? Why? 3. Read the text and fill in sentences (A–H) which best fit each paragraph (1– 6). There is one extra sentence you do not need to use. Experimental Design From Latin ex- + -periri (akin to periculum attempt). In the scientific method, an experiment is a set of actions and observations, performed to verify or falsify a hypothesis or research a causal relationship between phenomena. 1)□ Experimental design attempts to balance the requirements and limitations of the field of science in which one works so that the experiment can provide the best conclusion about the hypothesis being tested. 2) □ On the other hand, in other cases such as biology, and medicine, it is often hard to ensure that the conditions of an experiment be performed consistently; and in the social sciences, it may even be difficult to determine a method for measuring the outcomes of an experiment in an objective manner. For this reason, sciences such as physics are often referred to as "hard sciences", while others such as sociology are referred to as "soft sciences"; in an attempt to capture the idea that objective measurements are often far easier in the former, and far more difficult in the latter. 3) □ When the desire is to test a hypothesis that works "in general", an experiment may have a great deal of internal validity, in the sense that it is valid in a highly controlled situation, while at the same time lack external validity when the results of the experiment are applied to a real world situation. 4) □ As a result of these considerations, experimental design in the "hard" sciences tends to focus on the elimination of extraneous effects; while experimental design in the "soft" sciences focuses more on the problems of external validity, often through the use of statistical methods. 5) □ In such cases the problem of the scientist is to evaluate the natural "design". A In some sciences, such as physics and chemistry, it is relatively easy to meet the requirements that all measurements be made objectively, and that all conditions can be kept controlled across experimental trials. B One of the reasons why this may happen is because of the Hawthorne effect. C Occasionally events occur naturally from which scientific evidence be drawn, natural experiments. D In addition, in the soft sciences, the requirement for a "controlled situation" may actually work against the utility of the hypothesis in a more general situation. E The experiment is a cornerstone in empirical approach to knowledge. 4. Do you know the English names for any laboratory equipment? Match the following words, word combinations with pictures. Give Russian equivalents for them.
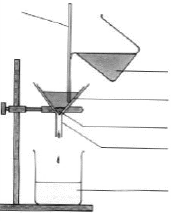
5. Look at the diagram Filtration. Complete the labels (a–f) using the words: filtrate, filter paper, mixture, residue, glass rod, funnel and make up sentences with them. Filtration
6 (a). Complete the table below using the extract from the following research paper to help you. A promising candidate among the different adsorbent materials is activated carbons. Through activation, highly porous materials can be prepared. Due to their high porosity, activated carbon materials are able to adsorb large amounts of hydrogen. Following adsorption, hydrogen molecules can be found at two possible locations: (i) on the surface of the adsorbent, or (ii) as a compressed gas in the void space between adsorbent particles.
6 (b). In pairs, answer the following questions. What noun suffixes are used in the words in the table? Can you think of other words with these suffixes? What adjective suffixes are used in the words? Can you think of other words with these suffixes? Why is it useful to know the suffixes for different parts of speech? 7. Fill in the appropriate word(s) from the list below into the text and then translate the sentences into Russian: prepared, to be provided, flask, collected, dissolve, warming, contact, laboratory, crucibles, flames, order, carry out, devices. At the Laboratory All the laboratories of inorganic chemistry are almost alike. These are large rooms where both students and research-workers ___1) their experimental work. Modern laboratories of inorganic as well as organic and analytical chemistry are provided with gas and running water. Every laboratory is ___2) with a ventilating hood for the escape of both harmful and unpleasant vapours and odours. Every laboratory has to be lit up very well. There are many laboratory benches with a great number of drawers in every laboratory. Different apparatus, ___3) as well as materials are to be kept in them. Besides we can see many shelves and cases for containers with chemicals. On every laboratory bench one can see test-tubes, flasks, beakers, funnels, evaporating dishes, weighing bottles. All this glassware should be kept in good ___4). Various burners serve for producing ___5). Bunsen burner is to be mentioned among them. Different crucibles are to be employed when heating of solution and igniting of materials are to be carried out. ___6) are usually made of quartz, porcelain and iron. In addition to these crucibles, there are platinum crucibles in some laboratories, but they are used very seldom. Many experiments can be carried out in the ___7) of inorganic chemistry. Thus, if we want to obtain hydrogen chloride (HCL) which is often referred to as a hydrochloric acid gas, it is necessary to pour some sulphuric acid through a tube over the crystal of sodium chloride, in a flask. The flask is to be heated. On ___8) the flask, the hydrogen chloride is expelled as a colourless gas with a suffocating odour. It produces heavy clouds of white fumes when it comes in ___9) with the moist air of the room. It is soluble and it cannot be ___10) over water as are oxygen and hydrogen. It is much heavier than the air and may be passed through a glass tube to the bottle. If we ___11) some of the gas in water, the solution has a sour taste, reddens blue litmus, reacts with zinc, etc.: it is hydrochloric acid. When all the sodium chloride originally present in the flask has been transformed, the reaction is complete. The ___12) then contains a salt called sodium acid sulphate (NaHSO4) together, with unchanged excess of sulphuric acid. Nitric acid may be ___13) by the reaction of concentrated sulphuric acid with sodium nitrate. In the laboratory method, a mixture of sodium nitrate and concentrated sulphuric acid is heated in a glass retort. Nitric acid is boiled out of the mixture and is condensed: NaNO3 + H2SO4 = HNO3+ NaHSO4. 8. Match the words with their definitions.
9. Describe one of chemical laboratories of your university. Talk about: experiments the students carry out what equipment every laboratory is provided with what it is necessary to do while working in a chemical laboratory 10. In pairs or small groups, make a documentary (or promotional video) about your university chemical laboratory. Present it in English to the class. Text 2 Active Vocabulary fade (v) – постепенно исчезать, расплываться, растворяться collect (v) – собирать stir (v) – мешать, помешивать, размешивать; взбалтывать cease (v) – переставать, прекращать settle (v) – оседать, осаждать(ся), отстаивать(ся) separate out from (v) – отделять(ся), выделять(ся), разделять(ся), сепарировать dissolve (v) – растворять(ся) disperse (v) – рассеивать(ся) solution (n) – раствор suspension (v) – суспензия 1. Chemical engineers often deal with different chemical processes and experiments. Study this diagram and the following passage carefully and then answer the questions. 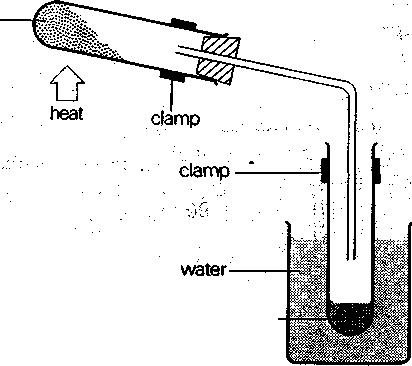 Experiment to show the presence of water of crystallization in a salt Experiment to show the presence of water of crystallization in a saltDry crystals of copper sulphate in the test-tube are heated. The colour of the crystals before heating is dark blue. When they are heated, the dark blue colour fades and the crystals turn white. At the same time, a colourless liquid is collected in the water-cooled tube. If the boiling-point and freezing-point of this liquid are measured, they are found to be the same as for water. If water is added to the cold dry white copper sulphate crystals, they turn blue and much heat is produced. This shows that blue copper sulphate crystals contain water, which is removed when the crystals are heated. 1. Describe the apparatus used in this experiment. 2. What is the colour of the crystals before heating? 3. What happens when the crystals are heated? 4. What tests are carried out with the liquid which is collected? 5. What do the tests show? |









 2
2  3
3  4
4
 6
6 7
7 8
8 
 10
10 11
11 12
12 
 14
14 15
15 16
16
 18
18 19
19 20
20 
 23
23
 26
26 27
27
 29
29 30
30