Английский. пособие Химики АЯ. Introduction
 Скачать 3.05 Mb. Скачать 3.05 Mb.
|
|
4 (b). Read the report again and answer the following questions. Why can we state that water passed up into the cylinders, but no protein solution passed into the water? At the end of the experiment, the height of the liquid in each cylinder was above the level of water in the beakers. What can be deduced from this about the membrane? What is the difference in pressure between the two columns of protein solution? What would happen if the membranes were punctured? What appears to have caused water to pass into the cylinders? Did water continue to pass into the cylinders throughout the experiment? What forces seem to be acting in this experiment? What seems to have happened at a certain point in the experiment? 5. When a report is made of an experiment, it is necessary to state what was seen, or noticed, or observed, etc. In the passage, the words observed, noticed, found, seen and noted were used. If a description is made of observations in the present, we can say: Bubbles of gas can be seen. or: A reaction is observed. or: Several substances are produced. However, if we were writing about the past, we would say: Bubbles of gas could be seen. A reaction was observed. Several substances were formed. Imagine you have carried out an experiment, and are writing up the report. Match the columns to make sentences.
6. When we look at results, we usually do so in order to reach conclusions. We examine the results and try to explain them. For example, When zinc is placed in dilute hydrochloric acid, bubbles can be observed coming from the zinc. We can therefore deduce: The bubbles must be due to a reaction taking place.The bubbles must be caused by a gas being given off. However, if we are writing about an experiment in the past, we must try to deduce the reason for what happened. For example, Zinc was placed in dilute hydrochloric acid. Bubbles could be observed coming from the zinc. This must have been due to a reaction. It must have been caused by a gas being given off.Make sentences about the observations and then state your deduction using the words and phrases in the table. bubbles of gas – carbon dioxide a pungent smell – hydrogen sulphide a heating effect – current ammonia – ammonia bubbles of gas – oxygen an effervescence – carbon dioxide a popping sound – bubbles of gas decrepitation – water
During the experiment, bubbles of gas could be observed. This must have been due to carbon dioxide being given off. 7. You can now describe the complete sequence of: 1. Procedure 2. Observation 3. Deduction 4. Reason. 1) Procedure: Zinc sulphide was placed in dilute sulphuric acid. 2) Observation: During the experiment, a pungent smell was noticed. 3) Deduction: This must have been caused by hydrogen sulphide being liberated. 4) Reason: ... because of a reaction between the zinc sulphide and the acid. Make similar statements with the following outlines. electric current/pass/coil of wire, heating effect current/pass/wire because/no other source of energy/available pieces/marble/place/dilute acid bubbles of gas carbon dioxide/evolved since/gas/turn/lime water/cloudy hydrogen peroxide/place/test-tube/with/manganese dioxide effervescence oxygen/liberated since/gas/cause/glowing splint/ignite copper sulphate crystals/heat/test-tube decrepitation water in crystals/expand due to/heat/give/crystals some chemicals/heat/test-tube popping sound bubbles of gas/explode since/bubbles of gas/observe/during/experiment slaked lime/and/ammonium chloride/heat pungent smell ammonia/evolved because/gas/cause/damp red litmus paper/turn/ blue 7. Fill in the appropriate word(s) from the list below into the text and then translate the sentences into Russian: increases, transfer, results, leading to, equal, proportional to, pressure, observed, present. The phenomenon ___1) during the experiment is called osmosis. This phenomenon and the ___2) of the experiment can be explained by considering what is happening in the liquid on either side of a semi-permeable membrane. On both sides of the membrane the molecules ___3) in the liquid will bombard the surface of the membrane. If on one side of the membrane there is a pure solvent and on the other a solution (as is the case for the experiment described), more solvent molecules will bombard the surface of the membrane on the pure solvent side than on the solution side. The membrane allows ___4) of the solvent molecules from one side to the other and prevents transfer of solute molecules. Thus, solvent molecules will transfer from the solvent to the solution simply because on that side of the membrane more solvent molecules bombard the surface, ___5) a greater number transferring across the membrane. Transfer will continue until the pressure exerted on the membrane by the solution (x and 2 x cm in the experiment) ___6) the number of solvent molecules bombarding the membrane surface in the solution up to a point when there are ___7) numbers of molecules transferring across the membrane from either side. The ___8) exerted under these conditions is called the osmotic pressure. The number of solvent molecules bombarding the membrane in the solution is directly ___9) the number of solute particles in the solution, hence the differing heights of solution in the experiment. 8 (a). In pairs, discuss the following questions. How do you usually present data of experiments? Why are visuals used in presenting data? What visuals do people in your field commonly use to show data? Why? 8 (b). Match the beginnings and endings of the sentences about setting data in tables and charts. Which advice do you think is the best? What other advice would you give to someone creating visuals to show data? Work with your partner to make a list of tips.
8 (c). Do you know what these visuals are called in English? Match the numbers to the descriptions. Then answer the questions. bar chart, table, technical drawing, flow chart, map, line graph, pie chart, organizational chart/ organigram.
Which type of visual is best to use ……… to show comparison between items? to describe a location? to show proportions of a whole? to describe a structure? to show trends? 8 (d). These verbs are used to describe movement or trends. Put them in the correct column: upward, downward or other form of movement. Make up sentences with these verbs.
9. Write a description of some visual data you are working on. 10. Make up a report of one of chemical experiments conducted at your university laboratory. Unit 4. Analytical Chemistry Text 1 Active Vocabulary attribute (n) – признак; показатель, характеристика, свойство spectrometry (n) – спектрометрия chromatography (n) – хроматография infrared (adj) – инфракрасный qualitative approach (phr) – качественный метод quantitative approach (phr) – количественный метод aim to (v) – нацеливаться embrace (v) – включать, заключать в себе, содержать, охватывать technique (n) – техника, методика, метод, способ involve in (v) – включать в себя 1. In pairs, discuss the following questions. 1. How would you define analytical chemistry? What is the scope of its study? 2. Is analytical chemistry concerned with a particular type of chemical compounds, like organic or inorganic chemistry? 3. What is the difference between qualitative and quantitative analysis? 2. Read the title of the text. What do you expect to read in the text? Read the text and check your guesses. What Is Analytical Chemistry? Analytical chemistry is the study of matter in order to reveal its composition, structure, and extent. Because these understandings are fundamental in just about every chemical inquiry, this field is used to obtain information, insure safety, and solve problems in many different chemical areas, and is essential in both theoretical and applied chemistry. Early analytical chemistry was mainly focused on identifying elements and compounds and discovering their attributes. Discovery gave way to systematic analysis, which took a giant step forward with the invention in the 1850’s of the first instrument for chemical analysis – flame emissive spectrometry – by Robert Bunsen, a German chemist who is better known for his invention of the Bunsen burner, and his colleague Gustav Kirchoff, a German physicist who is known for his 1862 coining the name "black body" radiation for an object that absorbs all of the electromagnetic radiation that reaches it. Other separation processes were developed, including various kinds of chromatography such as paper, gas, and liquid; electrophoresis; crystallography; microfiltration; and other spectrometers, including atomic absorption spectrometers, infrared spectrometers, and mass spectrometers. Other changes in the field took place, for example, the extension of analytical chemistry allowing for bioanalytical chemistry to develop. Bioanalytics includes areas such as genomics, lipidomics, metabolomics, peptidomics, proteomics, and transcriptomics. The traditional subdivisions of analytical chemistry followed the same paradigm as in statistical analysis: a qualitative approach that was focused on determining what elements and/or compounds were present and a quantitative approach that aimed to establish the precise amount of an element or compound in a given sample. Either, or both, of these approaches can be applied to materials in a variety of fields, including the food and beverage industry, the pharmaceutical industry, synthetic materials such as polymers, and natural materials, such as minerals and water samples. As the field grew, analytical chemistry also broadened to embrace applications of its techniques inforensics, and medicine. Analytical chemists today use a wide variety of techniques in their analyses, including some involving robotics, digital microscopes, a Fourier transform infrared spectophotometers, chip-based technology, and chemometrics, for example. They also use techniques in which technologies are combined, resulting in approaches referred to as hyphenated or hybrid techniques, characteristically referred to by initials. Examples include CE-MS–capillary electrophoresis-mass spectrometry; GC-MS–gas chromatography-mass spectrometry; CE-UV–capillary electrophoresis-ultraviolet; and HPLC/ESI-MS–high performance liquid chromatography/electrospray ionization-mass spectrometry. 3. Read the text again and answer the questions. What does analytical chemistry deal with? What was the focus of early analytical chemistry? What is bioanalytics? When was it developed? What two main types can analytical chemistry be split into? What does qualitative inorganic analysis seek? What is the aim of quantitative analysis? What techniques do analytical chemists use in their work? 4 (a). Give Russian equivalents for the following English words, word combinations and chemical terms. 1) study of matter; 2) extent; 3)fundamental; 4) chemical inquiry; 5) obtain information; 6) insure safety; 7) identifying elements; 8) discovering attributes; 9) take a giant step forward; 10) flame emissive spectrometry; 11) "black body" radiation; 12) electrophoresis; 13) crystallography; 14) microfiltration; 15) absorption spectrometers; 16) bioanalytical chemistry; 17) traditional subdivisions; 18) follow the same paradigm; 19) establish the precise amount of an element or compound. 4 (b). Give English equivalents for the following Russian words, word combinations and chemical terms. 1) основной; 2) опознавать, распознавать элементы; 3) изобретение; 4) создавать неологизмы; 5) поглощать электромагнитное излучение; 6) процесс разделения; 7) проба, образец; 8) расширять; 9) многообразие методов; 10) робототехника; 11) преобразование Фурье; 12) обеспеченный с помощью микросхем; 13) иметь результатом; 14) комплексные методы. 5. Fill in the appropriate word(s) from the list below into the text and then translate the sentences into Russian: burden, components, rates, analytical, instruments, seek, accuracy, developed, mass spectrometer, techniques. The importance of analytical chemistry has never been greater than it is today. The demand in modern societies for a variety of safe foods, affordable consumer goods, abundant energy, and labour-saving technologies places a great ___1) on the environment. All chemical manufacturing produces waste products in addition to the desired substances, and waste disposal has not always been carried out carefully. Disruption of the environment has occurred since the dawn of civilization, and pollution problems have increased with the growth of global population. The techniques of ___2) chemistry are relied on heavily to maintain a benign environment. The undesirable substances in water, air, soil, and food must be identified, their point of origin fixed, and safe, economical methods for their removal or neutralization ___3). Once the amount of a pollutant deemed to be hazardous has been assessed, it becomes important to detect harmful substances at concentrations well below the danger level. Analytical chemists___4) to develop increasingly accurate and sensitive techniques and instruments. Sophisticated analytic instruments, often coupled with computers, have improved the ___5) with which chemists can identify substances and have lowered detection limits. An analytic technique in general use is gas chromatography, which separates the different ___6) of a gaseous mixture by passing the mixture through a long, narrow column of absorbent but porous material. The different gases interact differently with this absorbent material and pass through the column at different ___7). As the separate gases flow out of the column, they can be passed into another analytic instrument called a ___8), which separates substances according to the mass of their constituent ions. A combined gas chromatograph–mass spectrometer can rapidly identify the individual components of a chemical mixture whose concentrations may be no greater than a few parts per billion. Similar or even greater sensitivities can be obtained under favourable conditions using ___9) such as atomic absorption, polarography, and neutron activation. The rate of instrumental innovation is such that analytic___10) often become obsolete within 10 years of their introduction. Newer instruments are more accurate and faster and are employed widely in the areas of environmental and medicinal chemistry. |

 1
1 2
2 3
3 4
4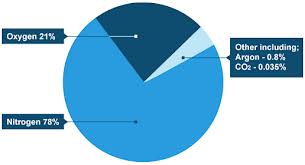 5
5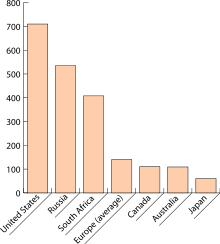 6
6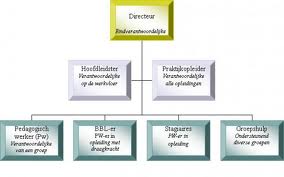 7
7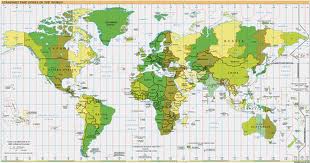 8
8