Английский. пособие Химики АЯ. Introduction
 Скачать 3.05 Mb. Скачать 3.05 Mb.
|
|
| 1. ∆ | a) about; approximately |
| 2. RT | b) at |
| 3. ± | c) because |
| 4. w/v | d) change |
| 5. @ | e) energy |
| 6. .’ . | f) increases |
| 7. . . ‘ | g) leads to |
| 8. → | h) more or less (to show the deviation from the number stated) |
| 9. ↑ | i) room temperature |
| 10 E | j) therefore |
| 11. w/ | k) weight per volume |
| 12. | l) with |
6 (b). In pairs, decide how you might represent each of the following in a lab notebook. What other abbreviations do you often use in your lab notebook?
| 1. decreases | 6. volume per volume |
| 2. degrees Celsius | 7. without |
| 3. kelvin | 8. two to one ratio |
| 4. greater than or equal to | 9. hours |
| 5. positive | 10. concentration |
7. In pairs, discuss the following questions.
What processes do you need to describe in your field of research? Who do you describe them for?
How much detail do you need to include in your descriptions?
What do you think are the most important points to remember when describing a process for other scientists?
How do you keep a record of your experiments?
Have you ever used a lab notebook software package?
How does the lab notebook protocol in your current lab compare to other labs you have worked in?
8. A сhemistry student is summarising an experiment he has run. The language in the summary is grammatically correct, but the style is inappropriate. Read the three extracts (A–C) below. Then in pairs, first find examples of inappropriate style in the extracts. Then discuss what the student could do to improve the style of his report.
   |
9. Vocabulary choice is very important to style. Below is a summary of another experimental procedure. For 1–10, underline the word or phrase which you think is in the most appropriate style for a formal scientific report.
In order to (1) determine / find out the value of n from (2) just one / a single experiment, (3) you should / it is necessary to have a range of stress levels actingwithin a (4) one thing / single specimen. (5) You can do this / This is achieved bymaking the sample into a coil. The stress (6) comes from / is provided by the weightof the coil itself, so that the upper part of the coil (7) experiences / gets more stressthan the lower parts. The stress in a particular turn of the coil is (8) proportional to/ changes at about the same speed as its number, N, where the turns are numberedbeginning from the bottom turn and ending at the top. The shear stress τ in each turn(9) is totally different / varies from zero at the centre of the turn (axis of the coil) toa maximum value at the edge of the coil. The average local strain rate (10) is thus / must be kind of related to the spacing between turns, s, and the time, t.
10. Write a description of a chemical experiment you have carried out recently. Use the words and phrases from previous exercises to help you.
Text 3
Active Vocabulary
make observations (phr) – делать наблюдения
notice (v) – замечать, обращать внимание
remain (v) – оставаться
take place (phr) – происходить, случаться, состояться, иметь место
require (v) – требовать(ся)
produce changes (phr) – вызывать изменения
draw a conclusion (phr) – делать вывод
conclude (v) – делать вывод, выводить заключение
state (v) – заявлять, утверждать, констатировать, формулировать
seem to (v) – казаться, представляться
account (n) – отчёт, сообщение, доклад
1. Think about an experiment you have done recently. Then in pairs, discuss 1–5.
Briefly describe the experimental process.
Explain what you predicted would happen.
Describe what actually happened.
Explain what you learned from the experiment.
Explain what you did as a follow – up to the experiment.
2. When the report of the procedure of an experiment is complete, it is then necessary to describe the results, including the observations which were made, and then draw conclusions from the results. Here is a description of what was observed during the experiment and a conclusion based on the observations and results. Study it and answer the questions about the results.
Reporting Observations and Results. Stating Conclusions
The water in the calorimeter was observed carefully as the temperature approached 0 °C. It was observed that the temperature continued to fall for a short time without any ice forming. The temperature was seen to fall a few degrees below 0 °C while the water remained liquid. It was then noticed that the temperature rose to 0 °C and ice began to form. The temperature was found to remain constant at 0 °C when ice was present. The behaviour of the temperature of the water can be seen from the graph.
From the results, it can be seen that the temperature fell a few degrees below zero before ice formed. The temperature then rose to zero as the ice formed. A loss of temperature indicates a loss of heat energy, therefore it can be stated that as the temperature of the water fell below zero, the water continued to give out energy to the surrounding freezing mixture. Since the temperature rose as the ice formed, it can similarly be stated that energy was gained by the water as it became solid.
When the water froze, a change of state took place, as the water changed from a liquid to a solid state. It was noted that the temperature rose during this time. Heat energy must therefore have been absorbed by the water during the change of state.
It can therefore be concluded that energy was required in order to bring about a change of state in the water. The experiment demonstrates that energy is required to produce a change of state.
1. What was observed when the temperature was around 0 °C?
2. When did ice begin to form?
3. What does a fall in temperature indicate?
4. Why is it possible to state that energy was absorbed by the water as it solidified?
5. The water changed from liquid to a solid. What can we say took place?
6. What must have happened in order to produce this change?
7. How do we know this?
8. What is the conclusion of the experiment?
3. Read the sentence: The temperature continued to fall for a short time, but no ice formed. This is another way of saying: The temperature continued to fall for a short time without any ice forming. Study the following situations and then re-phrase the sentences using without.
A metal wire was stretched 5 cm, but it didn't break.
Mercury was cooled to –20 °C, but it didn't freeze.
Sand was added to water and the mixture was heated gently for a long time, but the sand did not dissolve.
Some aluminium was placed in water and left for some time, but no reaction was observed.
A current was passed through a wire for a period of time, but no heating effect was detected.
A substance was heated for a short time, but no rise in temperature was observed.
Various substances were placed in diluted hydrochloric acid, but no gas was seen to be evolved.
Some ammonium chloride was heated, but no ammonia was smelt.
A lighted splint was held over the top of a test-tube of gas, but no explosion was heard.
A suspended magnet was brought near to a coil of wire, but no effect was noticed on the magnet.
4 (a). Read the report of the results of an experiment and put the paragraphs in the correct order.
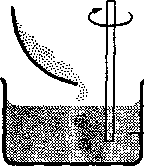
A) Since, at the end of the experiment, the level of solution in each cylinder was higher than the level of water in the beakers, it can be stated that the membrane must be capable of supporting a certain pressure of solution and preventing the solution from passing through it. Consideration of the diagrams showing the results of the experiment will show that there is a pressure of Xcm of solution acting on the membrane supporting the solution of concentration 5gdm‾3 and a pressure of 2Xcm acting on the membrane containing the solution of concentration 10gdm3. There will also be atmospheric pressure acting on both of the membranes.
B) If the membranes were punctured, the solutions would flow into the beakers until the levels of liquid inside and outside the cylinders were equal. It therefore seems that there is a driving force which causes the water molecules to pass through the membrane into the solution. However, the passage of water molecules through the membrane clearly stopped at some point when the column of solution reached a certain height. This can be explained as follows: as the column of solution rises up the cylinder, the pressure acting on the membrane will increase. When this pressure is equal to the driving force causing the water molecules to move across the membrane into the solution, a state of equilibrium is obtained. This means that there are "the same number of water molecules crossing the membrane from either side and so the height of the column of solution will not increase further.
C) It appears that water has been transferred through the membranes into the cylinders during the experiment, causing the levels of solution inside the cylinders to rise. Since the membranes used were semi-permeable, no protein could have passed into the solvent in the beakers. The movement was therefore from the solvent across the membrane into the cylinders.

 -water -
-water -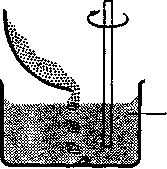 sand particles
sand particles